Project Description
Key information
Project in the Spotlight: E18017 (3DMed)
Market: Additive Biotech
Written by: Franz Bormann
The previous decade has been a time full of technological advancements in a large variety of industry sectors. One sector that has gained a growing interest and is constantly delivering a strong societal impact is the biotech industry. Empowered by new technologies and an ever-increasing computing power, year by year new opportunities arrive – such as the adoption of additive manufacturing (AM) for the delivery of personalized medical devices. Considering that most of the medical devices (surgical instruments, prostheses, orthoses, etc.) are still off-the-shelf products, or customised by experts in a time-consuming process, AM offers in the near future a great solution for rapidly manufactured and personalised medical devices. Yet, two major bottlenecks still prevail and prevent its widespread application: i) The high lead time from design to the printed product, and ii) the related high costs. To overcome these limitations, the 3DMed project was initiated.
Project objectives and outcomes
The 3DMed project was an Interreg 2 Seas program set up by M2i and coordinated by TU Delft. In a cross-border approach, top universities and innovative companies from the Netherlands, Belgium, France, and the UK joined forces to advance the potential of additive manufacturing (AM) in the medical care sector. In particular, the project’s overarching objective was the improvement of the affordability and the large-scale accessibility of medical treatment using 3D printed devices. The sub-goals of this project were:
- Technological advancement of metallic, composite and polymeric 3D printed medical devices.
- Delivery of personalized medical devices based on novel designs, and validation of their superior performance.
- Streamlined care by integrating diagnosis, design and manufacturing of 3D printed medical devices with pre- and post-operatory evaluation into an end-user friendly software platform.
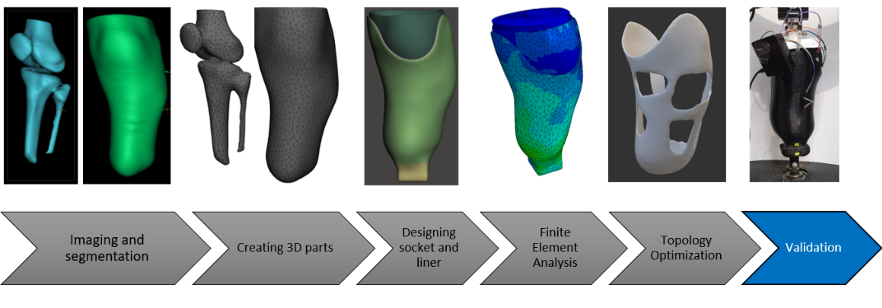
For this project a total of six demonstrators were chosen, covering protheses, orthoses, and surgical instruments. Four of these demonstrators (transtibial socket, hand brace, cast replacement and scoliosis brace) were to be designed on the basis of individual patient scans via the route of finite element (FE) simulations in combination with topology optimisation. The goal of the design optimisation was an increased wear comfort (via lower weight and an open design) while guaranteeing a high device functionality. That is where M2i’s role in the project as an FE expert came into play.
With the goal of maximising the practicality of the developed FE assisted software platform in an industrial setting, M2i defined and worked on three key focus areas:
- Reliability and high functionality of the newly designed medical device. With its modelling expertise, M2i adopted a consultant role for the academic partners to support the development of efficient and highly accurate demonstrator FE models.
- Fast product delivery. Utilising a submodelling approach for the demonstrator FE models, M2i demonstrated the feasibility of a tremendous computational speed-up (beyond 85%) of the topology optimisation simulations within design process.
- User-friendliness and ease of setting up the design process. M2i developed a plug-in for the semi-automated setup of one of the demonstrator FE models. The plug-in was successfully transferred to TU Delft, where it was extended to other demonstrator cases and implemented into the final software platform.
By May 31, 2022, the 3DMed project was concluded. Throughout its course it has greatly promoted the technology readiness level (TRL) of AM in the medical section (from 2-3 to 5-7). In addition, it delivered a streamlined software platform for the semi-automated design creation of 3D printed personalised medical devices within a routine clinical setting. Currently, M2i and TUD are exploring opportunities in form of follow-up projects to advance the developed software platform to a higher TRL.
 This research was carried out within the project “3DMed – Development and streamlined integration of 3D printing technologies to enable advanced medical treatment and its widespread application”, co-funded by the INTERREG 2SeasMers Zeeën programme http://www.interreg2seas.eu/3dmed
This research was carried out within the project “3DMed – Development and streamlined integration of 3D printing technologies to enable advanced medical treatment and its widespread application”, co-funded by the INTERREG 2SeasMers Zeeën programme http://www.interreg2seas.eu/3dmed
The 3DMed project enabled us to develop and validate 3D printing for medical applications, including tough and flexible materials, high quality surface finish and skin-safe colourants. With the submodelling approach designed by M2i and TU Delft, patient-specific designs can be ready for printing within minutes. Mechanical validation of the printed designs show that the devices are strong and perform as designed. This proves that additive manufacturing can be used for critically loaded applications as well. The gained knowledge and developed protocols will smoothen the implementation of 3D printing for medical devices. This would not have been possible without this multi-disciplinary collaboration.
Jan Deckers (VIGO, BE)
It was really a pleasure working together with technology leading partners such as M2i, TU Delft, Oceanz and Ghent University, while explorin g different aspects related to the digitalization of orthotic and prosthetic (O&P) devices. The optimized designs of the project demonstrators taught us how patient comfort could be optimized without sacrificing product reliability and functionality. Through innovative combination of 3D printing and post-processing technologies, Oceanz could create 3D printed materials that can compete with materials that are traditionally used within the O&P industry. At last, Ghent University created a beyond-the-state-of-the-art finite element model of the spine that enabled our future path towards evidence-based corsets.
g different aspects related to the digitalization of orthotic and prosthetic (O&P) devices. The optimized designs of the project demonstrators taught us how patient comfort could be optimized without sacrificing product reliability and functionality. Through innovative combination of 3D printing and post-processing technologies, Oceanz could create 3D printed materials that can compete with materials that are traditionally used within the O&P industry. At last, Ghent University created a beyond-the-state-of-the-art finite element model of the spine that enabled our future path towards evidence-based corsets.
Vahid Moosabeiki and Helda Pahlavani (TU Delft, NL)
It has been a great pleasure to be a part of the 3DMed project and to collaborate with the experienced industrial and academic partners. With the help of M2i, VIGO, Oceanz, and other partners, we were able to considerably expedite the design process for patient-specific medical devices, and to develop a user-friendly software platform to streamline the design workflow. With the established protocols and the workflows we are now one step closer to the personalized medical devices on which society can rely.